Researcher Must First Complete - at a Minimum - Basic TriNetX Training
A. REQUESTING UMMS EPIC DATA
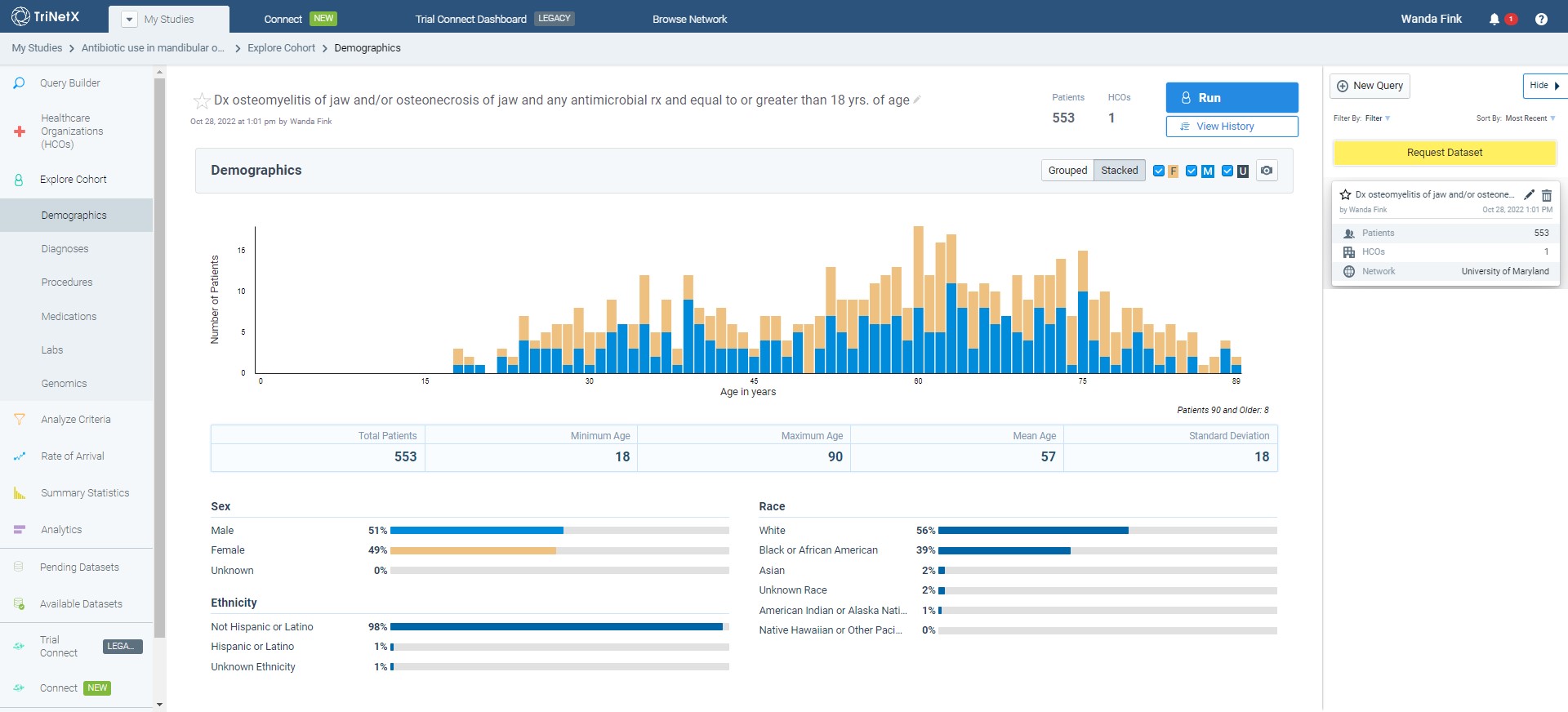
- We recommend you validate your query with TriNetX’s Query Design Assistance team to ensure your query accurately reflects your research study aims. They can be accessed via the “Design Assistance” button on the bottom left corner of the TriNetX Study Workspace. More information on the Query Design Assistance team can be found at this website (note that a TriNetX account will be needed to access this link): https://support.trinetx.com/hc/en-us/articles/34601096597787-What-is-Query-Design-Assistance.
- Once your query is validated, open the Query HistoryUMMS Research Informatics Core (RIC) Epic team panel on the right side of the Query Builder Tool and click the yellow “Request Dataset” button.
- Click on the “Available Datasets” tab on the navigation panel on the left side of the Study Workspace.
- The ICTR/UMMS Research Informatics Core (RIC) will be alerted and will follow up with you about the approvals needed to receive clinical data (IRB review as well as UMMS approval).
Suggested IRB language for approval to receive UMMS Epic Data
We will use the TriNetX Research Network database - a web-based tool for research population cohort and feasibility queries - that displays pooled, aggregate clinical data of dozens of academic medical centers and health care organizations (HCOs) that participate in TriNetX. The University of Maryland Medical System (UMMS) is one of the participating HCOs, allowing UMB researchers to view de-identified, aggregate data for cohort discovery.
We will validate our queries using the TriNetX’s Query Design Assistance team to ensure our queries accurately reflect our research study aims. Once we validate our queries, we will request record level data by submitting an ICTR Resource Request. The request is reviewed by the ICTR for completeness and then forwarded to the ICTR/UMMS Research Informatics Core (RIC) Epic team for consultation. Once the RIC team confirms IRB approval for the variables/domains needed for the study, the RIC team will move the record-level dataset to the UMB Secure Research Environment (SRE) Azure Virtual Desktop (AVD) for the PI of this protocol and the approved research team members on this IRB submission to access. No data can be moved out of this environment without UMMS approval. A data use or transfer agreement may be required,
More information about the UMB SRE and AVD can be found here https://www.umaryland.edu/cits/services/secure-research-environment-guidebook/. The SRE AVD is the required UMMS/UMB storage platform for TriNetX Research Network data downloads. The data cannot be moved from this environment without UMB and UMMS approval and other required approvals, such as Data Use Agreements from UMB/UMMS and assistance from the RIC team.
B. REQUESTING A TriNetX RESEARCH NETWORK DATASET
UMMS has joined over 90 healthcare organizations (HCOs) across the nation in allowing their Epic data to be pooled with the other participating HCOs. This is a limited dataset (includes dates of service and events) and you WILL NOT be able to identify the data's associated HCOs. Please note that UMB and UMMS require UMB IRB review to access TriNetX Research Network datasets. The IRB protocol must be specific to the research requiring the TriNetX Research Network data. Recommended IRB language is below.
We recommend you validate your query with TriNetX’s Query Design Assistance team to ensure your query accurately reflects your research study aims. This team can be accessed via the “Design Assistance” button on the bottom left corner of the TriNetX Study Workspace. More information on the Query Design Assistance team can be found at this website (note that a TriNetX account will be needed to access this link): https://support.trinetx.com/hc/en-us/articles/34601096597787-What-is-Query-Design-Assistance.
Once your query is validated, open the Query History panel on the right side of the Query Builder Tool and click the yellow “Request Dataset” button. The ICTR and the UMMS Research Informatics Core (RIC) Epic team will be notified. Additional Instructions:
- The PI of the project must email the ICTR-Navigator@umaryland.edu (cc EDA-Research@umm.edu as well) with the following information:
a. A brief description of the research aims and the purpose of the TriNetX Research Network dataset.
b. Whether the study is grant-funded or non-grant-funded. Requests for datasets for non-grant-funded projects are provided at no cost. For grant-funded projects, TriNetX will negotiate a fee with the PI.
c. How long you would like access to the dataset (the default is 1 year).
d. By default, TriNetX provides a limited data set (contains dates of service and events). If you wish the project to be considered Not Human Research for your IRB submission, UMMS team can modify/remove these elements to deidentify the dataset before making it available to you.
We will forward this information to TriNetX who will then reach out to the PI of the project directly to review and complete a Data Set Order Form, which outlines the Terms of Use for the dataset.
TriNetX will make the data available to the RIC team to move from the TriNetX space and into the UMB Secure Research Environment (SRE) Azure Virtual Desktop (AVD). This is where the PI and the research team noted on the IRB approved protocol will be able to view and analyze.
Suggested IRB submission language for TriNetX Research Network Data Projects
We will be querying the TriNetX Research Network database, a web-based tool for research population cohort and feasibility queries that displays pooled, aggregate Epic clinical data of over 90 participating healthcare organizations (HCOs) across the nation. The University of Maryland Medical System (UMMS) is one of the participating HCOs. The pooled data may be obtained through the process noted below. At most, only limited datasets (only includes dates of service and events) or a de-identified dataset (all 18 HIPAA identifiers removed) can be provided through the process noted below. For the purposes of this study, the PI will be requesting a [choose one: limited dataset or de-identified dataset (all 18 HIPAA identifiers removed)]
To obtain the dataset, the PI will request the data from TriNetX who will follow up with the PI to discuss the purpose of the data request. With IRB review and approval, TriNetX will work with the UMMS/UMB Institute for Clinical & Translational (ICTR) Research Informatics Core (RIC) (aka Honest Broker) to provide the dataset to the RIC team who will then move it into the UMB Secure Research Environment (SRE) Azure Virtual Desktop (AVD) for the PI of this protocol and the IRB-approved research team to access.
More information about the UMB SRE and AVD can be found here https://www.umaryland.edu/cits/services/secure-research-environment-guidebook/. The SRE AVD is the required UMMS/UMB storage platform for TriNetX Research Network data downloads. The data cannot be moved from this environment without UMB and UMMS approval and other required approvals as needed, such as Data Use Agreements from UMB/UMMS and assistance from the RIC team.