‘Rolling Stone’ Profiles UMB Vaccine Efforts
The unprecedented work of University of Maryland, Baltimore (UMB) researchers is getting the rock star treatment, gracing the pages and pixels of Rolling Stone.
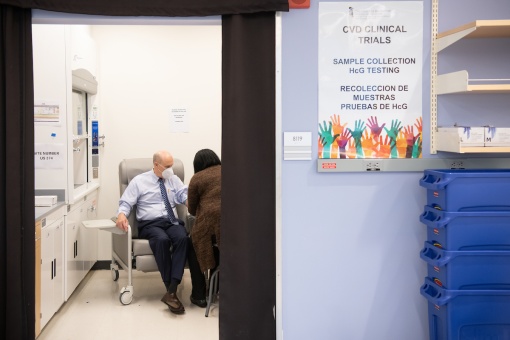
This photo of UMB President Bruce E. Jarrell, MD, FACS, appeared in Rolling Stone. Here, Jarrell is participating in the Moderna mRNA vaccine trial on his first day as president. Matthew Paul D'Agostino / UMB
The Rolling Stone long read looks at a snapshot in time of what it’s like working on a COVID-19 vaccine using months-long interviews for “Inside the Race to End the Pandemic.”
The writer interviewed a team at the University of Maryland School of Medicine’s (UMSOM) Center for Vaccine Development and Global Health (CVD), which has been at the forefront of finding a vaccine or treatment for COVID-19 since the pandemic began here in March.
Appearing in the article are:
- Wilbur H. Chen, MD, MS, associate professor of medicine, chief of the Adult Clinical Studies section within the CVD, and director of the UMB Travel Medicine Practice.
- Lisa Chrisley, RN, BSN '92, clinical research nurse manager for CVD
- Kirsten Lyke, MD, professor of medicine and director of the Malaria Vaccine and Challenge Unit.
- Kathleen Neuzil, MD, MPH, FIDSA, Myron M. Levine, MD, DTPH, professor in vaccinology and director of CVD.
- Kathleen Strauss, CVD laboratory specialist.
The web version of the article prominently displays monotypes created by Strauss incorporated in a photo illustration, in addition to photos of UMB President Bruce E. Jarrell, MD, FACS, and UMSOM graduate student David Rach receiving injections for COVID-19 vaccine trials.
The doctors, scientists, and staff have participated in studying two vaccines to combat COVID-19: the Pfizer, Inc. and BioNTech Phase One trial, of which UMSOM gave their final dose in July, and the Moderna, Inc. Phase Three trial that is now being conducted.
One of the Pfizer vaccine candidates is moving forward to the final stage of clinical trials.
The Moderna trial is the first to be implemented under Operation Warp Speed, a multi-agency collaboration led by the U.S. Department of Health and Human Services that aims to accelerate the development, manufacturing, and distribution of medical countermeasures for COVID-19.
In addition, the UMSOM team has studied two potential treatments, including the drug remdesivir.
The author takes readers through the emotional toll for the researchers as they treat patients one minute and work on a vaccine the next. All the while, regulations, political discourse, the well-being of the researchers and personal loss are woven into the narrative for a publication that counts 76 percent of its audience between 18 and 49 years old.
“I mean, you cannot make a mistake during these [trials]. My biggest problem has been calming down,” Strauss was quoted as saying in the story, which detailed her creative outlet of monotypes as a stress reliever.
What might go unappreciated in the article is that UMB is one of a select few institutions in the country able to handle research and clinical trials. The article notes the distinction that CVD is a Vaccine Testing and Evaluation Unit for the National Institutes of Health (NIH). As such, the CVD participates in Phase 3 trials for the NIH-funded vaccine.
Lyke distinctly notes that testing multiple vaccines is not a competition among the vials.
“I would definitely term it as a ‘race against the virus’ and, in no way, a race against any other vaccine strategies or teams,” Lyke told Rolling Stone. “I think all of us hope that many, many vaccine development teams are successful given the complexities of manufacturing and the sheer numbers of doses required.”
You can read more about CVD’s efforts to find a vaccine for COVID-19 at Rolling Stone’s website.



