Round Up of CVD's Typhoid Efforts: Typhoid Mary to Typhoid Conjugate Vaccines, H
.png)
Typhoid Mary to typhoid conjugate vaccines – how far we have come
In the early 1900s, cook Mary Mallon – also known as Typhoid Mary – infected 50 to over 100 people with typhoid. As she changed jobs, she took typhoid with her from household to household. While typhoid is a disease of the past in developed countries, it still plagues many in low- and middle-income countries. The Typhoid Vaccine Acceleration Consortium (TyVAC) – led by the Center for Vaccine Development and Global Health (CVD) – has joined forces to Take on Typhoid.
A recently published Typhoid Fever Nature Reviews Primer and blog – co-authored by CVD’s Dr. Kathleen Neuzil and TyVAC partners – provides a comprehensive overview of the epidemiology, disease burden, risk factors, genomics, and challenges, including multidrug resistance, diagnostics, and clinical presentation, as well as recent advances in the vaccine pipeline, clinical trial data, and the potential for disease prevention and reduction.
Typhoid, a serious and sometimes fatal bacterial infection is caused by Salmonella enterica serovar Typhi. Typhoid is spread through contaminated food and water, and feces. In 2019, more than 9 million cases and 110,000 deaths were attributed to typhoid. Luckily, there is a safe and cost-effective typhoid conjugate vaccine (TCV). Pakistan, Samoa, Liberia, Nepal, Zimbabwe – and recently Malawi – have successfully introduced TCVs into their national immunization programs. Taking on typhoid requires a coordinated that includes improved diagnostics and water, sanitation, and hygiene infrastructure. TCVs can help shape a future where typhoid is a disease of the past.

Reaching Zero-Dose Children in Nepal
 In April 2022, Nepal vaccinated more than 7 million children during a national campaign to introduce typhoid conjugate vaccine (TCV). The national campaign was also an opportunity to identify and reach zero-dose (ZD) children – those younger than 2 years old who have not received any routine vaccinations - or who are under-immunized. Trained healthcare workers used vaccination cards to track children with missed doses and offered guidance to caregivers, advising them to visit the nearest health facility to access missed vaccines. The outreach strategies and lessons learned through TCV introduction provide a roadmap for others working to reach ZD children and missed communities, ensuring that all children are protected from typhoid.
In April 2022, Nepal vaccinated more than 7 million children during a national campaign to introduce typhoid conjugate vaccine (TCV). The national campaign was also an opportunity to identify and reach zero-dose (ZD) children – those younger than 2 years old who have not received any routine vaccinations - or who are under-immunized. Trained healthcare workers used vaccination cards to track children with missed doses and offered guidance to caregivers, advising them to visit the nearest health facility to access missed vaccines. The outreach strategies and lessons learned through TCV introduction provide a roadmap for others working to reach ZD children and missed communities, ensuring that all children are protected from typhoid.
The Typhoid Vaccine Acceleration Consortium (TyVAC), led by UMSOM’s Center for Vaccine Development and Global Health, works to decrease the burden of typhoid by accelerating the introduction of TCVs in high-risk countries.
Typhoid conjugate vaccine provides sustained protection beyond 4 years
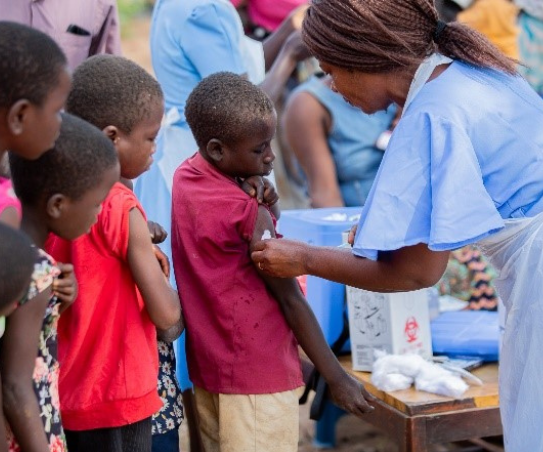 Researchers at the Malawi-Liverpool-Wellcome Trust confirmed typhoid conjugate vaccines (TCVs) prevent blood culture-confirmed typhoid for over 4 years among children 9 months-12 years.
Researchers at the Malawi-Liverpool-Wellcome Trust confirmed typhoid conjugate vaccines (TCVs) prevent blood culture-confirmed typhoid for over 4 years among children 9 months-12 years.
In this study, children were randomly assigned to receive either a single dose of TCV or meningococcal capsular group A conjugate (MenA) vaccine. The research team conducted passive surveillance across four sites, where vaccinated participants were tested for typhoid fever and other pathogens if they presented with a fever. Over 7 months, a total of 24 children in the TCV group and 110 children in the MenA group had a positive blood culture for typhoid. The overall vaccine efficacy of TCV after more than 4 years was 78.3%, with an estimated 1.3% decline in efficacy per year.
As emphasized in the accompanying commentary, a single dose of TCV is both safe and well-tolerated, demonstrating effectiveness in protecting against typhoid. These longer-term data are pivotal for decision-makers in prioritizing the introduction of TCVs.
The Typhoid Vaccine Acceleration Consortium (TyVAC), led by UMSOM’s CVD, works to address the disease burden by accelerating the introduction of TCVs in high-risk countries.


